A Comprehensive Guide to ICP-MS Technology and Applications
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) is a highly sensitive analytical technique that is used to determine the trace and ultra-trace levels of elements in a sample. The method is widely used in various applications such as environmental monitoring, food safety, and biomedical research. This technology has revolutionized the way we approach elemental analysis and has become an essential tool for scientists and researchers in a variety of fields.
Background:
This technology was first developed in the 1980s and has since become an essential tool in a variety of fields, including environmental monitoring, food safety, and biomedical research.

ICP-MS operates by ionizing a sample and analyzing the resulting ions using mass spectrometry. This allows for the precise determination of trace and ultra-trace levels of elements in a sample. It is widely considered to be one of the most sensitive and accurate methods of elemental analysis available, making it a crucial tool for scientists and researchers.
When compared to other elemental analysis techniques, such as atomic absorption spectrophotometry (AAS) and graphite furnace atomic absorption spectrometry (GFAAS), ICP-MS has several key advantages. These include higher sensitivity, wider dynamic range, and the ability to analyze a greater number of elements simultaneously. Additionally, ICP-MS is a multi-elemental analysis method, meaning it can be used to analyze many elements in a single run, providing a comprehensive analysis of a sample.
In this comprehensive guide, I will delve into the working principle of ICP-MS, the key components of an ICP-MS system, and its applications. I will also discuss the advantages and limitations of ICP-MS and examine the future of the technology. Whether you are a scientist or researcher or simply interested in learning about ICP-MS, this guide will provide you with the information you need to fully understand and appreciate the technology.
Working Principle
ICP-MS is a highly sensitive analytical technique used to determine the elemental composition of a sample. This technique is an elemental analysis, meaning it measures elements rather than the molecules and compounds measured by LC/MS and GC/MS.
The process of ICP-MS involves converting the sample into ions using an argon plasma, known as the ICP, which is then measured using a mass spectrometer.
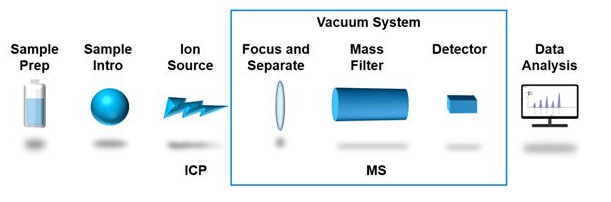
An ICP-MS instrument includes the ion source, a mass spectrometer, and a detector. The ICP operates at atmospheric pressure while the mass spectrometer and detector operate in a vacuum chamber, requiring a vacuum pump, vacuum interface, and ion lenses to focus the ions through the system.
Sample Introduction
The process begins by introducing a sample into an inductively coupled plasma (ICP) source. The ICP source is created by applying a high-frequency electromagnetic field to an argon gas, which results in the ionization of the gas and the formation of a plasma.
The sample introduction system pumps the sample from the vial into the nebulizer, where it is mixed with Argon gas to form an aerosol. This aerosol then passes through the cooled spray chamber, which converts the liquid sample into a fine mist.
This mist is then introduced into the ICP.
Plasma Generation
Once the sample has been introduced into the ICP, the next step is to generate the plasma. This is done by passing an electrical current through the coil surrounding the ICP. The electrical current generates a magnetic field, which then induces a secondary current in the sample, vaporizing it. The resulting high-temperature, high-density plasma is then used to excite the sample's atoms, producing positively charged ions.
Mass Analysis
The resulting dried, decomposed, atomized, and positively charged ions are then analyzed using a mass spectrometer. In a typical ICP-MS system, the ions are first passed through a skimmer cone, which helps to remove any residual neutral species and to focus the ion beam. The ion beam is then passed through into the high vacuum region containing the ion lenses and mass analyzer.
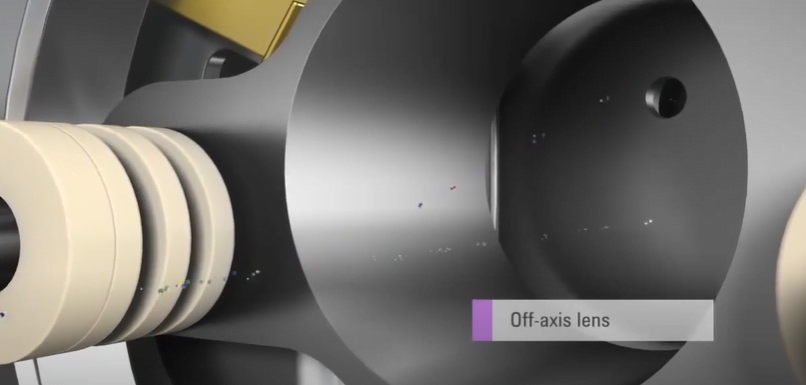
The mass analyzer used in ICP-MS can be either a magnetic sector analyzer or a quadrupole analyzer. In a magnetic sector analyzer, the ions are separated based on their m/z ratio as they pass through a magnetic field. In a quadrupole analyzer, the ions are separated based on their m/z ratio as they pass through a series of multiple electrodes. The resulting electronic signals are processed and stored in a multichannel scalar, creating a mass spectrum where the intensity at a given mass is proportional to the concentration of the isotope at that mass.
Systems such as Agilent 7500 feature octopole reaction system technology and helium collision mode. The octopole reaction cell uses an octopole ion guide rather than the hexapoles and quadripoles used in other systems, allowing for higher pressure operation and greater interference removal efficiency.
In the helium collision mode, polyatomic interferences collide with helium atoms and lose kinetic energy, allowing only the higher energy analyte ions to pass through to the mass analyzer. This mode removes all polyatomic species simultaneously without the need for prior knowledge of the sample matrix. This model is also inert, not reacting with the analyte or sample matrix, making it suitable for multi-element analysis in complex sample matrices. With its rapid and accurate semi-quantitative analysis capability, the 7500 can measure more than 60 elements in a completely unknown sample in just one minute without the need to run calibration standards.
Finally, the separated ions are detected, and their signal is processed by a detector. In most ICP-MS systems, the detector is an electrostatic analyzer, which measures the ion current produced by the ions as they pass through the analyzer.
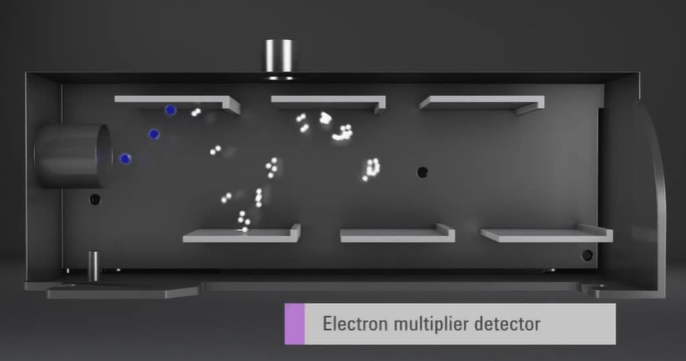
The resulting data is then processed using software, which generates a mass spectrum that displays the relative abundances of the various elements in the sample.
Key components of an ICP-MS system include the ICP source, the skimmer, the mass analyzer, the detector, and the software for data processing. The ICP operates at atmospheric pressure while the mass spectrometer and detector operate in a vacuum chamber, requiring a vacuum pump, vacuum interface, and ion lenses to focus the ions through the system. In addition to the vacuum pump, an ICP-MS system also requires a chiller to regulate the temperature of the instrument, ensuring that it remains stable and consistent
These components work together to ensure the precise and accurate analysis of elements in a sample.
Sample Preparation for ICP-MS Analysis
Sample preparation is a critical aspect of ICP-MS analysis. In order to obtain accurate results, it is important to understand what types of samples are suitable for ICP-MS and how they should be prepared.
ICP-MS is capable of analyzing a wide range of sample types, including liquids, solids, and gases. However, the sample must be in a form that can be introduced into the ICP-MS instrument for analysis. This means that solid samples must be dissolved in a solution, and gaseous samples must be transformed into a liquid or solid form.
Once the sample is in the appropriate form, it must be properly prepared for analysis. This typically involves diluting the sample to an appropriate concentration, filtering it to remove any particulate matter, and adding any necessary stabilizing agents or matrix modifiers. Proper sample preparation is essential to avoid matrix effects, which can impact the accuracy of the results.
In summary, sample preparation is a crucial step in ICP-MS analysis, and it is important to understand what types of samples are suitable for ICP-MS and how they should be prepared to ensure accurate results.
Applications:
ICP-MS is widely used in a variety of applications, due to its high sensitivity and accuracy. Some of the key applications of ICP-MS include:
- Environmental Monitoring: ICP-MS is commonly used in environmental monitoring to determine the levels of toxic elements in soil, water, and air samples. The technology is also used to monitor the levels of nutrients and contaminants in food and agricultural products.
- Food Safety: ICP-MS is an important tool for ensuring food safety, as it can be used to determine the levels of contaminants, such as heavy metals, in food and food products.
- Biomedical Research: ICP-MS is used in biomedical research to determine the levels of elements in biological samples, such as blood and tissue. This information is crucial for understanding the role of elements in human health and disease.
- Geological Research: ICP-MS is used in geological research to determine the elemental composition of rocks, minerals, and other geological samples. This information is used to understand the formation and evolution of the earth and its environment.
- Industrial Analysis: ICP-MS is used in a variety of industrial applications, including the analysis of electronic components, catalysts, and other industrial materials.
The key benefits of ICP-MS in each of these applications include its high sensitivity, accuracy, and multi-elemental analysis capabilities. These benefits have made ICP-MS an essential tool for scientists and researchers in a variety of fields.
In the next section, we will discuss the advantages and limitations of ICP-MS, as well as its comparison to other elemental analysis techniques.
Advantages and Limitations:
Like all analytical techniques, ICP-MS has both advantages and limitations. Some of the key advantages of ICP-MS include:
- High Sensitivity: ICP-MS is one of the most sensitive methods of elemental analysis available, allowing for the detection of trace and ultra-trace levels of elements in a sample.
- Wide Dynamic Range: ICP-MS has a wide dynamic range, allowing for the analysis of elements at concentrations ranging from parts-per-billion (ppb) to parts-per-million (ppm).
- Multi-Elemental Analysis: ICP-MS is a multi-elemental analysis technique, meaning it can be used to analyze many elements in a single run. This provides a comprehensive analysis of a sample.
- High Accuracy: ICP-MS is highly accurate, providing precise and reliable results.
- Versatility: ICP-MS can be used in a wide variety of applications, including environmental monitoring, food safety, biomedical research, and industrial analysis.
- Speed of Analysis: ICP-MS is known for its speed of analysis, making it a useful tool in industries such as pharmaceuticals, where quick analysis of raw materials is critical. With its high sensitivity and multi-element analysis capability, ICP-MS can provide fast and reliable results in a matter of minutes.
However, there are also some limitations to ICP-MS, including:
- Complexity: ICP-MS is a complex technology that requires a high level of technical expertise to operate and maintain.
- Cost: ICP-MS systems are relatively expensive, making the technology inaccessible to some researchers and institutions.
Despite these limitations, the future of ICP-MS is bright. Advances in technology are expected to improve the accuracy and speed of analysis while making the equipment more accessible and user-friendly. The integration of ICP-MS with other analytical techniques, such as Liquid Chromatography (LC) and Gas Chromatography (GC), is also expected to enhance its capabilities. These advances will likely continue to shape the future of ICP-MS and its impact on various industries.