
A Comprehensive Guide to Chromatography and HPLC / LC-MS
Chromatography is a separation technique used in analytical chemistry to identify, isolate, and quantify individual components in a mixture. The history of chromatography dates back to the early 20th century when the Russian botanist Mikhail Semenovich Tswett first used it to separate plant pigments. Today, chromatography is a widely used technique in a variety of industries, including biotechnology, pharmaceuticals, and food and beverage production.

There are various types of chromatography, each of which is designed to handle different sample matrices and separation challenges. Some of the most common types of chromatography include gas chromatography (GC), high-performance liquid chromatography (HPLC), and liquid chromatography-mass spectrometry (LC-MS).
In this article, we will explore the different types of chromatography, their applications, and the advantages and disadvantages of each technique. By the end of this article, you will have a better understanding of the different types of chromatography and how they are used in various industries.
Basic Chromatographic Methods
Chromatography is a versatile analytical technique used to separate and identify components of a mixture. There are various chromatographic methods, but some of the most commonly used are paper chromatography, column chromatography, and Thin Layer Chromatography (TLC).
- Paper Chromatography Paper chromatography is one of the earliest forms of chromatography and is still used today as a simple and inexpensive way to separate components of a mixture. In this method, a mixture is applied to a piece of chromatography paper and then the paper is placed in a solvent. As the solvent moves up the paper, it carries the components of the mixture with it and they separate based on their interaction with the paper and the solvent. This technique is often used to identify the components of a mixture and determine their relative proportions.
- Column Chromatography Column chromatography is another popular chromatographic method. In this technique, the mixture is applied to a column filled with a stationary phase, which can be a solid or a liquid. The stationary phase is chosen based on the properties of the components in the mixture and their desired separation. A solvent is then passed through the column, and as it moves through the stationary phase, it carries the components with it, resulting in their separation. This method is often used for larger-scale separations and purification of compounds.
- Thin Layer Chromatography (TLC) Thin Layer Chromatography (TLC) is a quick and simple chromatographic technique used to separate and identify components of a mixture. In this method, a mixture is applied to a thin layer of silica gel or alumina on a glass plate. The plate is then placed in a solvent, which moves up the plate and carries the components with it. As the solvent reaches the top of the plate, the components will separate based on their interaction with the stationary phase. This method is often used as a quick screening method to determine the components of a mixture and their relative proportions.
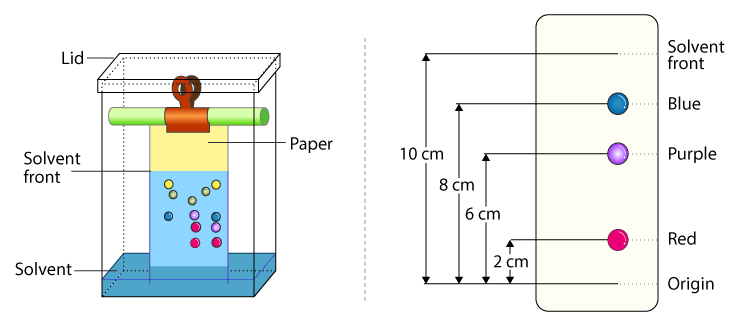
These three chromatographic methods offer a range of techniques for separating and identifying components of a mixture. Each method has its own advantages and disadvantages, and the choice of method will depend on the desired separation, the properties of the components, and the amount of sample available.
High-Performance Liquid Chromatography (HPLC)
High-Performance Liquid Chromatography, commonly referred to as HPLC, is a popular form of liquid chromatography that is widely used in a range of analytical applications. It involves the separation of analytes based on their physical and chemical properties, with the aim of producing a pure and well-defined sample that can be analyzed further.
The working principle of HPLC
The working principle of HPLC is relatively simple. A sample is first injected into the HPLC system and then it is separated into its individual components as it passes through a column filled with a stationary phase. The separation of the sample occurs as a result of the different interactions between the components of the sample and the stationary phase.
Comparison with HPLC and other analytical techniques
Compared to other chromatographic techniques such as column chromatography and thin-layer chromatography (TLC), HPLC offers several advantages. It is highly sensitive and capable of separating a wide range of compounds with high efficiency. This is particularly important for complex samples that contain multiple components, as HPLC is able to separate and purify each component individually.
HPLC is also capable of providing accurate and reproducible results, making it a useful tool for the quantification of analytes. In addition, HPLC can be used with a range of detectors, including UV-Vis, fluorescence, and mass spectrometry, which enables the analysis of a wide range of samples with different chemical properties.
HPLC and LC-MS (Liquid Chromatography Mass Spectrometry) are both popular analytical techniques used for the separation and analysis of complex samples. While both use liquid chromatography as a separation technique, LC-MS adds the ability to identify and quantify individual compounds through mass spectrometry analysis. In other words, HPLC is mainly used for separations, while LC-MS combines separations with identification and quantification capabilities.
Liquid Chromatography Mass Spectrometry (LC-MS)
Definition of LC-MS
LC-MS is a type of analytical instrument that is used to identify and quantify small and large molecules in complex mixtures. This technique is a combination of liquid chromatography (LC) and mass spectrometry (MS). The LC component of LC-MS separates the components of a sample based on their physical and chemical properties, and the MS component identifies and quantifies the components based on their mass-to-charge ratio.
Working principle of LC-MS
The LC component of LC-MS uses a high-pressure pump to force a sample through a column filled with a stationary phase. The stationary phase is chosen based on the properties of the sample components and the desired separation. The sample is separated into its individual components as it travels through the column, and the separated components are then detected by the MS component.
In the MS component of LC-MS, the separated components are introduced into an ionization source, where they are converted into ions. The ions are then passed through a mass analyzer, which separates the ions based on their mass-to-charge ratio. The separated ions are then detected and quantified by a detector.
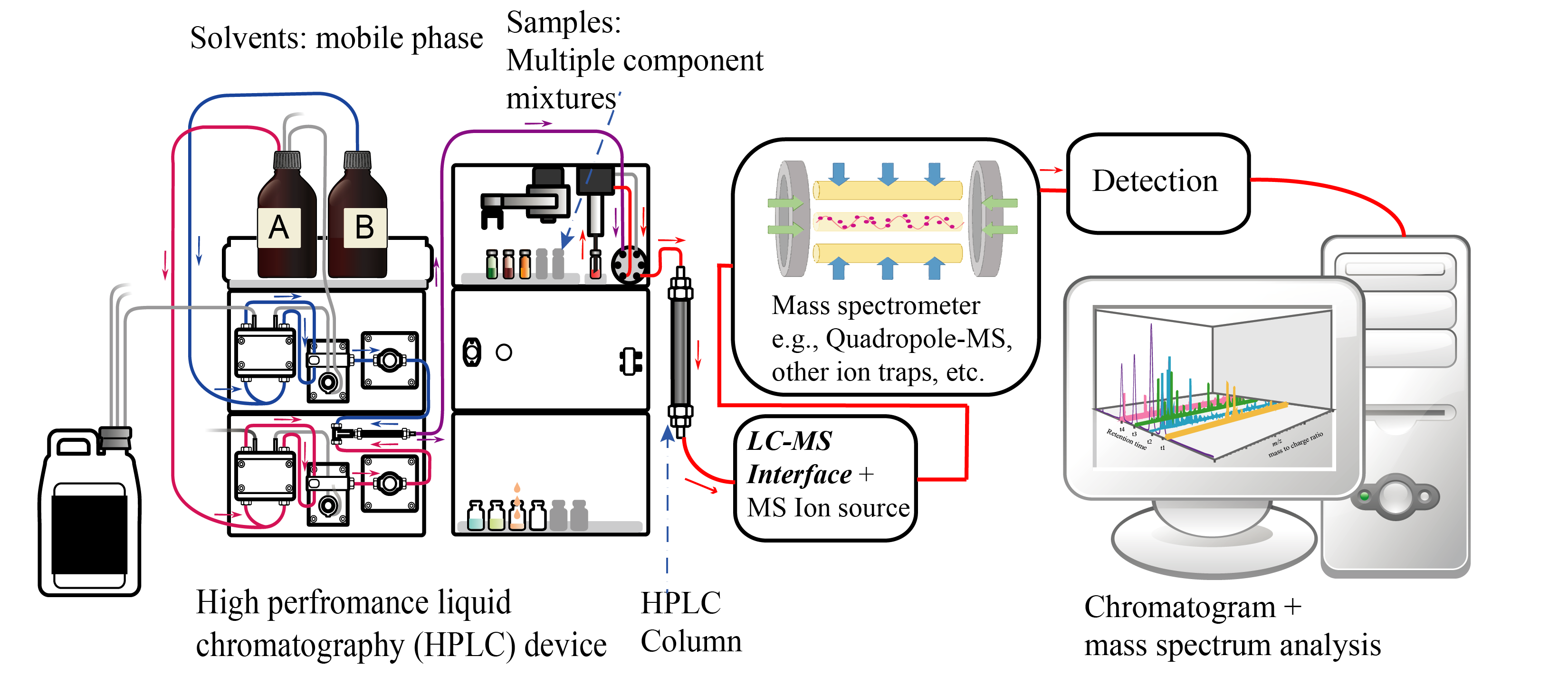
Comparison with HPLC and other analytical techniques
LC-MS offers many advantages over traditional chromatographic techniques such as HPLC. One of the main advantages of LC-MS is its ability to identify and quantify both large and small molecules in a sample. This is because the mass spectrometry component of LC-MS can detect ions with a wide range of masses. LC-MS also offers a higher level of sensitivity and specificity compared to traditional chromatographic techniques.
Additionally, LC-MS offers improved selectivity compared to other analytical techniques, as it can separate and identify individual components in complex mixtures. This makes LC-MS ideal for applications in the pharmaceutical and biopharmaceutical industries, where the analysis of complex mixtures is often required.
Advantages and Limitations of LC-MS
- High sensitivity and precision - LC-MS is a highly sensitive and precise analytical technique, making it an ideal choice for applications that require precise quantification of trace amounts of compounds. The mass spectrometry aspect of LC-MS allows for the detection of very small quantities of a substance, and the chromatography aspect of LC-MS provides the selectivity and specificity needed to distinguish between different compounds.
- Matrix independence - LC-MS provides matrix independence, meaning that it can accurately quantify compounds in complex matrices such as biological fluids, environmental samples, and industrial process streams. The chromatography aspect of LC-MS allows for the separation of compounds from matrix interferences, while the mass spectrometry aspect provides the sensitivity and specificity needed for accurate quantification.
- Speed of analysis - LC-MS is a fast and efficient analytical technique, providing results in a matter of minutes to hours. This makes it ideal for high-throughput analytical applications and the analysis of large sample sets.
Challenges in LC-MS analysis
Despite its many advantages, LC-MS analysis is not without its challenges. One of the main challenges is the need for sample preparation, which can be time-consuming and may introduce additional sources of error. Additionally, the high sensitivity of LC-MS can sometimes lead to interference from matrix components, requiring additional steps to ensure accurate quantification. Finally, the cost of LC-MS equipment and the need for trained personnel can be significant limitations for some applications.
Despite these challenges, LC-MS remains a highly valuable analytical tool for a wide range of applications, providing the sensitivity, specificity, and speed of analysis needed for the accurate quantification of compounds in complex matrices.
Applications of LC-MS
- Analysis of Environmental Contaminants - The LC-MS technique has been used to analyze a wide range of environmental contaminants, including heavy metals, pesticides, and other toxic substances. For example, the technique has been used to study the cause of Chronic Kidney Disease of Unknown Origin (CKDu) in Sri Lanka.(Aflatoxins)
- Analysis of Biomolecules - LC-MS is widely used in the analysis of biomolecules, including proteins, peptides, nucleic acids, and lipids. For example, the technique has been used to analyze the proteome of yeast cells, providing insights into cellular processes and functions. The LC-MS analysis was used to identify and quantify the different proteins present in the yeast cell extract.
- Analysis of Small and Large Molecules - LC-MS is suitable for the analysis of both small and large molecules, making it a versatile technique. For example, LC-MS has been used to analyze the metabolism of small molecules such as drugs, vitamins, and hormones. It has also been used to analyze large molecules such as proteins and carbohydrates.
- Analysis of Pharmaceuticals and Biopharmaceuticals - LC-MS is widely used in the pharmaceutical industry for the analysis of drugs and biopharmaceuticals. For example, LC-MS has been used to analyze the purity and potency of biological drugs, such as monoclonal antibodies and vaccines. The LC-MS analysis was used to identify and quantify the different components of the biological drug, including impurities and degradation products.
Column Selection in LC-MS
Types of Columns used in LC-MS
- Reverse Phase Columns: These columns are based on hydrophobic interactions and are used for the separation of polar and non-polar compounds.
- Ion Exchange Columns: These columns are based on ionic interactions and are used for the separation of charged molecules such as proteins, peptides, and nucleic acids.
- Size Exclusion Columns: These columns are based on molecular size and are used for the separation of large biomolecules such as proteins and peptides.
- Hydrophilic Interaction Chromatography (HILIC) Columns: These columns are based on hydrophilic interactions and are used for the separation of polar compounds.
- Aqueous Normal Phase (ANP) Columns: These columns are based on polar interactions and are used for the separation of non-polar compounds.
- Affinity Columns: These columns are based on specific chemical interactions between the analyte and a specific ligand and are used for the purification of specific proteins or molecules.
Examples: C18 and C18 columns 
Factors to Consider when Selecting Columns
When it comes to selecting columns for LC-MS analysis, there are several factors to consider in order to ensure optimal results. Here are some of the key factors to consider when making your selection:
A. Type of Sample
The type of sample being analyzed will play a significant role in determining which column to use. For example, if you are analyzing highly polar or basic samples, you may want to consider using a reverse phase column, as these columns are specifically designed to retain polar and basic compounds. On the other hand, if you are analyzing non-polar or acidic samples, an ion-exchange column may be a better choice.
B. Polarities of Analytes
Another important factor to consider when selecting columns for LC-MS is the polarity of the analytes you are trying to detect. Polar analytes will generally interact more strongly with reverse phase columns, while non-polar analytes will interact more strongly with ion-exchange columns. It is important to choose a column that will provide optimal retention and separation of your target analytes.
C. Column Performance and Stability
Finally, you will want to consider the overall performance and stability of the column you are considering. Some columns may have a more robust and stable packing, which can be important for longer analysis times or when analyzing complex samples. In addition, it is important to choose a column that has been thoroughly validated and has a proven track record of performance in similar applications.
By considering these factors, you can select the best column for your LC-MS analysis and ensure that you get the most accurate and reliable results.
Detectors used in HPLC and LC-MS
There are several detectors that can be used with HPLC, including Diode Array Detector (DAD) and Variable Wavelength Detector (VWD). The choice of detector depends on the type of analysis being performed and the specific requirements of the sample
- Diode Array Detector - The Diode Array Detector (DAD) is a type of UV-Vis spectrophotometer that can simultaneously measure the UV spectrum of multiple compounds in a sample. This allows for the rapid identification of compounds based on their unique UV spectrum. DADs are commonly used in HPLC for the analysis of pharmaceuticals, food and beverage samples, and other complex mixtures.
- Variable Wavelength Detector- The Variable Wavelength Detector (VWD) is another common detector used in HPLC. Unlike the DAD, the VWD measures the sample at one specific wavelength at a time, allowing for more precise quantification of individual compounds. VWDs are commonly used for the analysis of environmental samples and other complex mixtures where precise quantification is required.
- Mass Spectrometry (MS) Detectors: Mass spectrometry detectors use the mass-to-charge ratio of analytes to detect their presence. The way this works is that the analytes are ionized and the mass-to-charge ratio of the ions is measured. This measurement is used to determine the concentration of the analytes in the sample.
- Fluorescence Detectors: Fluorescence detectors are also commonly used in chromatography. These detectors use the fluorescent properties of analytes to detect their presence. The way this works is that the analytes are exposed to an excitation light source and the fluorescence that is emitted by the analytes is measured.
- Refractive Index (RI) Detectors: Refractive index detectors are used to detect analytes based on the change in refractive index of the sample as it moves through the column. The way this works is that the sample is passed through a column and the change in the refractive index of the sample is measured as it moves through the column.
- Electrochemical Detectors: Electrochemical detectors are used to detect analytes based on the change in electrical conductivity of the sample as it moves through the column. The way this works is that the sample is passed through a column and the change in electrical conductivity of the sample is measured as it moves through the column.
These are some of the different types of detectors that can be used in chromatography. Each type of detector has its own advantages and disadvantages and the best detector for a particular application will depend on the sample being analyzed and the analytical requirements.
Comparison between HPLC and LC-MS
Advantages and Limitations
High Performance Liquid Chromatography (HPLC) is a commonly used technique in the analysis of complex mixtures. It offers several advantages such as high resolution, high precision and the ability to separate a wide range of compounds. However, it has its limitations as well, for example, it is not capable of providing information about the molecular structure of the compounds.
Liquid Chromatography Mass Spectrometry (LC-MS), on the other hand, offers a unique advantage over HPLC. It combines the advantages of high separation power of liquid chromatography with the high sensitivity and specificity of mass spectrometry. It is capable of providing molecular structure information and quantitative data. However, LC-MS is more complex, time-consuming and expensive compared to HPLC.
Factors to Consider when Choosing between HPLC and LC-MS
When choosing between HPLC and LC-MS, several factors need to be considered, including the nature of the sample, the required information and the budget. If the primary objective is to separate complex mixtures and the molecular structure information is not necessary, then HPLC might be the better choice. On the other hand, if molecular structure information is required, then LC-MS should be considered.
HPLC uses a high pressure pump to move a sample mixture through a column filled with a stationary phase. The column separates the components of the mixture based on their physical and chemical properties. Detectors used in HPLC include Diode Array Detector (DAD) and Variable Wavelength Detector (VWD).
LC-MS is a combination of liquid chromatography and mass spectrometry. LC-MS is used to separate and identify complex mixtures by first separating the components using liquid chromatography and then analyzing them using mass spectrometry. LC-MS has high sensitivity and precision, is matrix independent, and has a fast speed of analysis. However, there are some challenges in LC-MS analysis, including the complexity of the instrumentation, the need for specialized expertise, and the high cost of the instrument.
When choosing between HPLC and LC-MS, the choice should be based on the specific needs of the analysis and the resources available. HPLC is a simpler and less expensive technique, but it is not as sensitive or precise as LC-MS. On the other hand, LC-MS is a more sensitive and precise technique, but it is more complex and expensive.
Conclusion
Chromatography and LC-MS are essential tools in analytical chemistry. They provide critical information about the composition and structure of chemical mixtures, allowing for the identification and characterization of individual components. Their importance in a wide range of scientific and industrial applications highlights the need for continued development and innovation in these techniques.
