
Harnessing Nature for a Cleaner Tomorrow: Activated Carbon/Chitosan Hybrid Material for Lead Removal
Research Article Details
- Title: Fabrication of Activated Carbon/Chitosan Hybrid Material for Adsorptive Removal of Pb (II)
- Journal name: CHEMISTRY & CHEMICAL TECHNOLOGY (Chem.Chem.Technol.; Сh&ChT)
- DOI: https://doi.org/10.23939/chcht17.04.903

Introduction
The issue of toxic metal contamination poses a silent yet insidious threat to our drinking water. My focus as a Sri Lankan researcher is on addressing this problem using waste materials. I’m actively developing a novel material known as activated carbon/chitosan hybrid material (ACCHM), which merges activated carbon(rice husk) and chitosan(shrimp shells). This innovative purification material holds significant promise in offering a safer, more cost-effective, and cleaner approach to water purification. The published research article explores the preliminary study of this particular material.
Objectives of the Study:
The research I conducted aimed to achieve several critical objectives in the field of water purification and heavy metal removal. Specifically, the objectives were:
- To Synthesize a Novel Adsorbent Material:
The primary goal was to develop a new type of adsorbent that could effectively remove heavy metals from drinking and wastewater. This adsorbent, activated carbon/chitosan hybrid material (ACCHM) was designed to be both cost-effective and environmentally friendly. - To Utilize Biomass Waste Products:
In synthesizing ACCHM, it was crucial to utilize readily available and low-cost natural resources. I chose rice husk for its carbon-rich content and “Black Tiger” shrimp shells for their chitin, which upon conversion, yields chitosan. These materials were selected not only for their functional properties but also to contribute to a waste-to-wealth initiative. - To Enhance the Adsorption Capacity:
The study sought to combine the advantages of activated carbon’s large surface area with chitosan’s unique binding properties. The intent was to create a material with enhanced pore structure and functional groups to improve the adsorption of toxic metals. - To Offer a Sustainable Alternative to Commercial Activated Carbon:
As coal-based activated carbon resources rapidly diminish, there’s a crucial need to envision an alternative. The goal is to provide a sustainable, biomass-derived activated carbon that isn’t reliant on non-renewable coal sources for its production. - To Assess the Performance of ACCHM:
An essential part of the study was to test and quantify the adsorption performance of ACCHM. This included determining the optimal conditions for Pb(II) removal and analyzing the kinetics of adsorption. - To assess the Activation performance by deviating from common practice
In diverging from common practices, a crucial aspect of our research involved using hydrochloric acid (HCl) as the activating agent instead of the more commonly used phosphoric acid (H3PO4) during the synthesis of the activated carbon component. Additionally, we achieved successful carbonization without using a nitrogen flow in the furnace by optimizing the conditions.
The rationale for this choice stems from several considerations:
- Availability and Cost-Effective Nature of HCl: HCl is a more readily available chemical reagent and generally more cost-effective compared to H3PO4. This aligns with our objective of creating a more affordable water purification process.
- Environmental and Handling Concerns: HCl has a lower environmental impact and is easier to handle during the production process due to its less viscous nature and the relatively straightforward neutralization process when compared to phosphoric acid.
Through these objectives, the research paper reflects a holistic approach to tackling the pressing problem of heavy metal contamination in water. By offering a scalable, cost-effective, and eco-friendly solution, the aim is to make a significant impact on improving water quality and public health on a global scale.
Methodology
In our research, we designed a method to synthesize the activated carbon/chitosan hybrid material (ACCHM) and assess its effectiveness in removing Pb(II) ions through adsorption. Below, you’ll find a detailed account of the different stages involved in our process:
Extraction and Characterization of Chitosan:
The initial step involved extracting chitosan from “Black Tiger” shrimp shells. The process began with demineralization, where shrimp shell powder was immersed in a 7% (v/v) HCl solution to remove minerals. This was followed by deproteinization using NaOH, and then dehydration with ethanol. We transformed the resulting chitin into chitosan via an alkaline treatment using NaOH at 110 °C . The degree of deacetylation of chitosan was determined using a specific calculation involving the absorbance of particular bands in the chitosan’s spectrum. (Equation 01 – See article)
Characterization techniques such as Scanning Electron Microscopy and Energy Dispersive Spectroscopy (SEM/EDAX), as well as Fourier Transform Infrared Spectroscopy (FTIR), were employed to analyze the physical and chemical properties of chitosan.
Preparation of Activated Carbon (AC) Derived from Rice Husk:
We then turned our attention to the activated carbon derived from rice husk. The rice husks were first carbonized at 400 °C, for varying durations to optimize the process. Subsequently, the husks were chemically activated using a hydrochloric acid (HCl) solution. The yield of the activated carbon was determined, and the sample with the highest Pb(II) removal efficiency was selected for further characterization with techniques similar to those used for chitosan.
Synthesis of Activated Carbon/Chitosan Hybrid Material (ACCHM):
To create the hybrid material, acid-treated activated carbon (HCl (5% v/v) for 24 h) was combined with the chitosan gel. The mixture was stirred at an elevated temperature (50 °C) , ensuring thorough integration of both components to form ACCHM. This material was further washed to neutrality and dried, followed by characterization using SEM/EDAX and FTIR.
Batch Adsorption Studies:
Finally, we conducted batch adsorption studies to determine the Pb(II) ion removal efficiency of ACCHM. The different initial metal ion concentrations were tested to evaluate the material’s maximum adsorption capacity. (2.10. Page 905)
These studies helped us understand how ACCHM performs under various conditions, paving the way for practical applications in water purification systems.
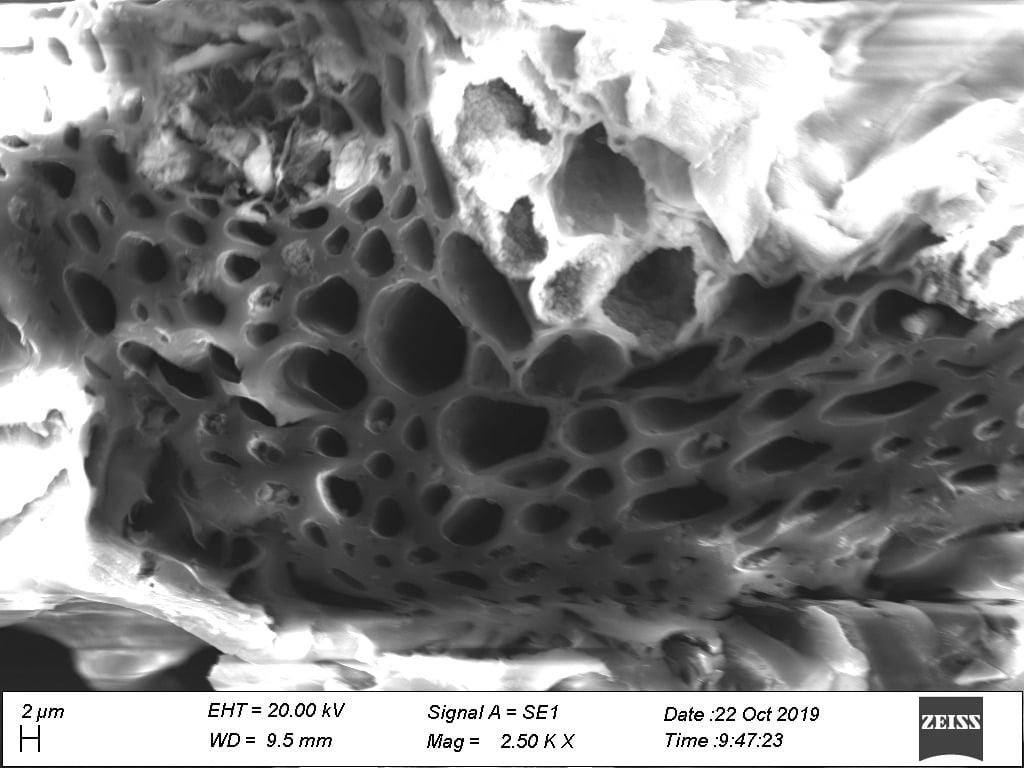
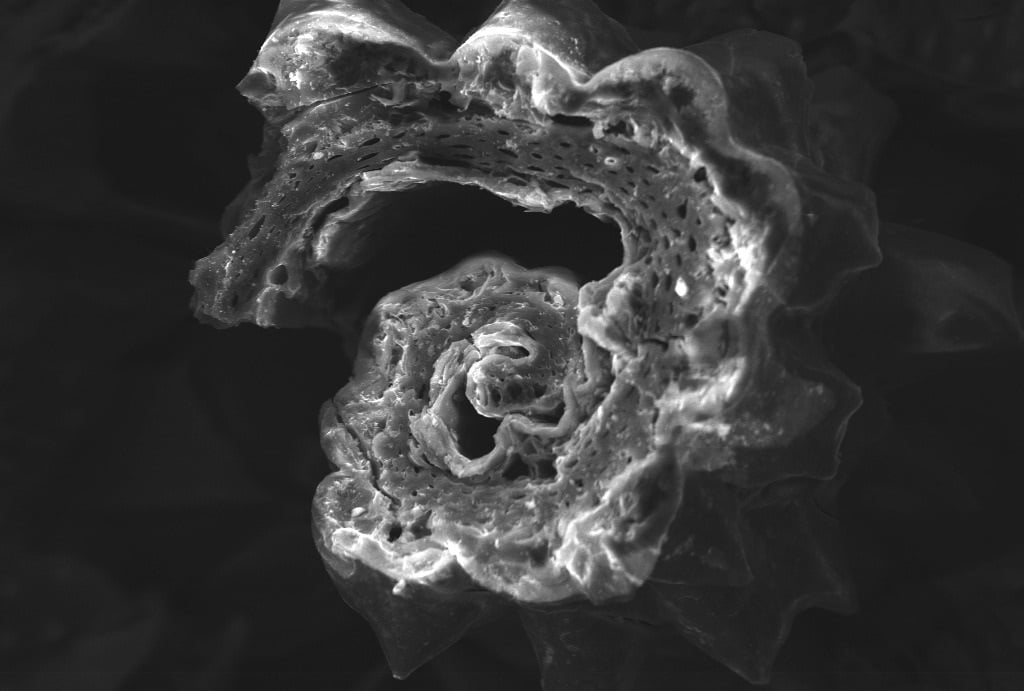
SEM – Revealing the Surface Morphology:
SEM helped to visualize the surface morphology of the ACCHM. The SEM images revealed a heterogeneous surface with an uneven texture characterized by an assortment of pores, indicating the activation process’s effectiveness in creating the porous structure essential for adsorption. This physical topology is inherently beneficial as it provides a significant amount of surface area for heavy metals like Pb(II) to adhere.
FTIR – Uncovering the Functional Groups:
FTIR spectroscopy was utilized to identify the chemical functional groups present on the surface of the chitosan, activated carbon, and ACCHM. Here are some notable findings from FTIR analysis:
- Chitosan Characterization: FTIR analysis of chitosan demonstrated bands at 1655 cm-1 and 1595 cm-1, attributed to the amide group’s stretching vibrations I and II, respectively. The presence of these bands supports the successful creation of chitosan with groups capable of binding metal ions.
- Activated Carbon Characterization: Activated carbon derived from rice husk showed a broad peak at 3435 cm-1, related to O-H stretching vibrations, and other key peaks associated with stretching vibrations of C=C and C=O groups. These oxygen-containing groups play a critical role in metal adsorption, largely due to their ability to form complexes with lead ions.
- ACCHM Analysis: In the hybrid material, FTIR spectra revealed similar bands associated with both activated carbon and chitosan, providing evidence that both components’ intrinsic properties were retained in the final ACCHM product. Notably, compared to the individual components’ spectra, there was a good representation of all functional groups involved, which suggests that the hybridization process was successful.
Through SEM, we discovered that the ACCHM possessed a synergistic structural framework conducive to high adsorption capacity. Concurrently, FTIR analysis elucidated that the diverse functional groups critical for chelating and binding lead ions were present and intact, further affirming the material’s expected efficacy in water purification tasks.
The combination of these techniques and their findings played a crucial role in verifying the anticipated performance benefits of the ACCHM, supporting the hypothesis that such materials can serve as valuable allies in the fight against water-based lead contamination.
Conclusion – A Leap Forward:
The conclusions drawn from our investigation underscore the success of ACCHM as a promising material for the adsorptive removal of Pb(II). Our research presented conclusive evidence that the integration of activated carbon with chitosan leads to a material that is better suited for tackling the challenges of lead contamination in water than either of the two components independently. ACCHM’s superior performance is attributed to its unique combination of physical and chemical properties that harness the strengths of both activated carbon’s porosity and chitosan’s metal-binding functionalities.
Contribution of the Research:
This project has made a significant contribution to the field of water purification by:
- Introducing a novel, sustainable adsorbent material that utilizes waste biomass, aligning with environmental and economic paradigms.
- Demonstrating a method to enhance the natural capabilities of activated carbon through hybridization with biopolymers such as chitosan.
- Providing a potential blueprint for industry applications in developing cost-effective and efficient water purification systems.
The Path Ahead:
While our study marks an advancement in water purification technology, it also lays the groundwork for further exploration within the academic community. It opens up a myriad of possibilities for subsequent research, such as:
- The study of the long-term stability and regeneration potential of ACCHM.
- The exploration of ACCHM’s adaptability and efficacy in various real-world water treatment contexts.
- The potential modulation of the synthesis process to tailor the material for the removal of other heavy metal contaminants.
Invitation to Scholars:
To fellow scholars, researchers, and practitioners in water purification, environmental science, and materials chemistry: I extend an invitation to explore the complete research paper for deeper insights into our work. I believe the findings hold valuable perspectives that can enrich your own research endeavors. I encourage you to cite and expand upon our study in your work.
By collaborating, we can push the limits of environmental sustainability and public health security, advancing solutions for clean and safe drinking water on a global scale. Thank you for engaging with this article, and I eagerly anticipate how this research will progress and expand through your scholarly contributions.
Publication Experience
Timeline
Submission: 21 Aug 2022
Reviews: 1 Oct 2022
Preliminary Accepted: 28 Oct 2022
Accepted: 08 Feb 2023
Published: 25 Dec 2023
Notes:
This journal based in Ukraine. Despite the ongoing situation, this journal consistently upholds clear communication even amid the country’s challenges. I highly recommend considering this journal for your publications. However, please note that the publication process might take approximately a year. Nevertheless, it offers open access and is free of charge.
